Baren-Boym Company helped Angiodynamics to design and develop Solero* Microwave Tissue Ablation (MTA) System. The Solero MTA System features the Solero Microwave (MW) Generator and the specially designed Solero MW Applicators. The solid state Solero MW Generator with a 2.45 GHz operating frequency can power up to 140 W for optimized power delivery and fast ablations. The Solero MW Applicator’s optimized ceramic tip diffuses MW energy nearly spherically, and its patented cooling channel with thermocouple provides real-time monitoring to help protect non-targeted tissue ablation. In addition, the Solero MTA System offers physicians scalability with a single applicator designed for multiple, predictable ablation volumes by varying time and wattage. 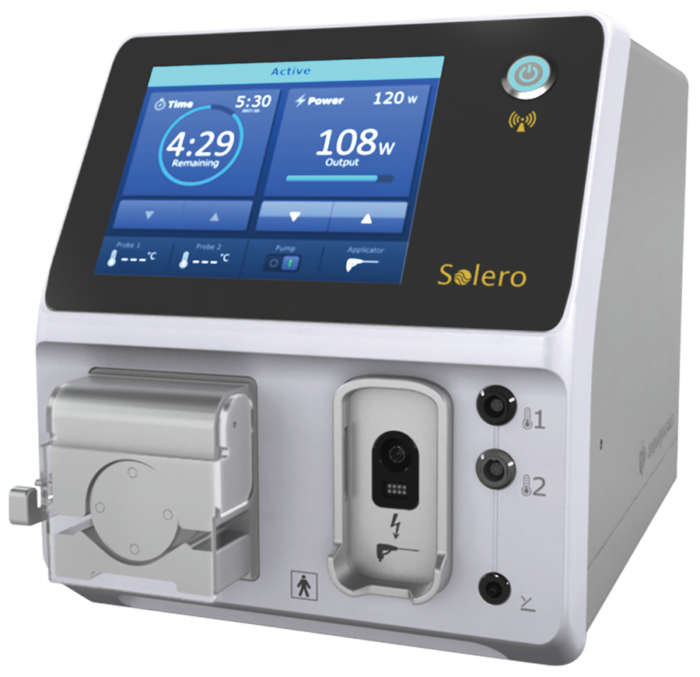
Baren-Boym Design: Disposable Digital Flexible Ureteroscope – May 1st, 2016
Baren-Boym Company has worked together with Boston Scientific to design a new disposable digital flexible ureteroscope for diagnosing and treating stones and other issues within the bladder, ureter, and kidneys. Being a single-use device, there’s no need for cleaning and sterilization, reprocessing, and management of the whole process. Moreover, reusable devices frequently break down and need expensive repairs. The LithoVue comes sterilized and pre-calibrated, brand new and ready to go for each procedure. The device can flex 270° in both directions and focus in on objects between 2mm and 50mm from the CMOS light sensor at the tip.
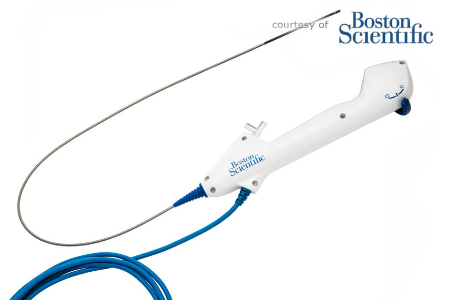 The LithoVue’s has its own display, but it can also be connected to the operating room’s own displays for more convenient visualization. The system is now being made available in the U.S., Europe, and New Zealand.
The LithoVue’s has its own display, but it can also be connected to the operating room’s own displays for more convenient visualization. The system is now being made available in the U.S., Europe, and New Zealand.
Baren-Boym Design: Versago Vascular Access Raises $1.65MM – April 12, 2016
 WEST BRIDGEWATER, Mass., Feb. 24, 2016 /PRNewswire/ — Versago Vascular Access Inc. (www.versagovascularaccess.com), developer of the world’s first “reverse needle” subcutaneous access port, has closed $1.65MM in seed funding from Primo Medical Group’s network of individual and institutional investors. Versago Vascular Access, a spinoff of Primo Medical Group and Baren-Boym Company designed and developed a new subcutaneous port to access the body’s bloodstream and anatomical cavities that collect serous or ascitic fluid. The technology provides a large bore, reusable, power injectable conduit directly into a patient’s bloodstream or other anatomical areas and in certain applications could potentially replace current technologies like ports, peripherally inserted central catheters (PICC) and central venous catheters (CVC) for a myriad of procedures. The company’s new funding will be used to further develop the Versago line of port technology, initiate regulatory review and conduct animal testing. Versago will also be initiating discussions with potential strategic partners in early 2016.
WEST BRIDGEWATER, Mass., Feb. 24, 2016 /PRNewswire/ — Versago Vascular Access Inc. (www.versagovascularaccess.com), developer of the world’s first “reverse needle” subcutaneous access port, has closed $1.65MM in seed funding from Primo Medical Group’s network of individual and institutional investors. Versago Vascular Access, a spinoff of Primo Medical Group and Baren-Boym Company designed and developed a new subcutaneous port to access the body’s bloodstream and anatomical cavities that collect serous or ascitic fluid. The technology provides a large bore, reusable, power injectable conduit directly into a patient’s bloodstream or other anatomical areas and in certain applications could potentially replace current technologies like ports, peripherally inserted central catheters (PICC) and central venous catheters (CVC) for a myriad of procedures. The company’s new funding will be used to further develop the Versago line of port technology, initiate regulatory review and conduct animal testing. Versago will also be initiating discussions with potential strategic partners in early 2016.
Boston Globe – Engineering His New Life – February 3, 2008
Michael Barenboym has designed a heart-assist device and a machine that processes bone marrow for use in bone-grafting procedures, and has improved on the no-drip valve mechanisms in toddler sippy cups. “A good mechanical engineer can design anything from an airplane to a child’s toy,” said the 41-year-old Framingham resident. “As long as they know the principles of aerodynamics, heat transfer, and strengths of materials.” Barenboym has spent evenings analyzing baby bathtubs to ensure that they will never tip over, and days designing a catheter, complete with a tiny camera, that can be steered on its ventures inside the body. Life is good for Michael Barenboym. He’s living the American dream and is not ashamed to say that, when he arrived in the United States from Russia in 1990, his first mattress was pulled from someone’s curbside trash…
Read more
